

Silver nitrate (AgNO 3) is light sensitive and is used to make photographic films and papers. High capacity batteries can be made with silver and zinc and silver and cadmium. Sterling silver, an alloy containing 92.5% silver, is used to make silverware, jewelry and other decorative items. Silver has also been used to create coins, although today other metals are typically used in its place. Silver is also the best reflector of visible light known, but silver mirrors must be given a protective coating to prevent them from tarnishing.

Pure silver is the best conductor of heat and electricity of all known metals, so it is sometimes used in making solder, electrical contacts and printed circuit boards. A chemical element, often simply called an element, is a type of atom which has the same number of protons in its atomic nucleus (i.e., the same atomic number, or Z).
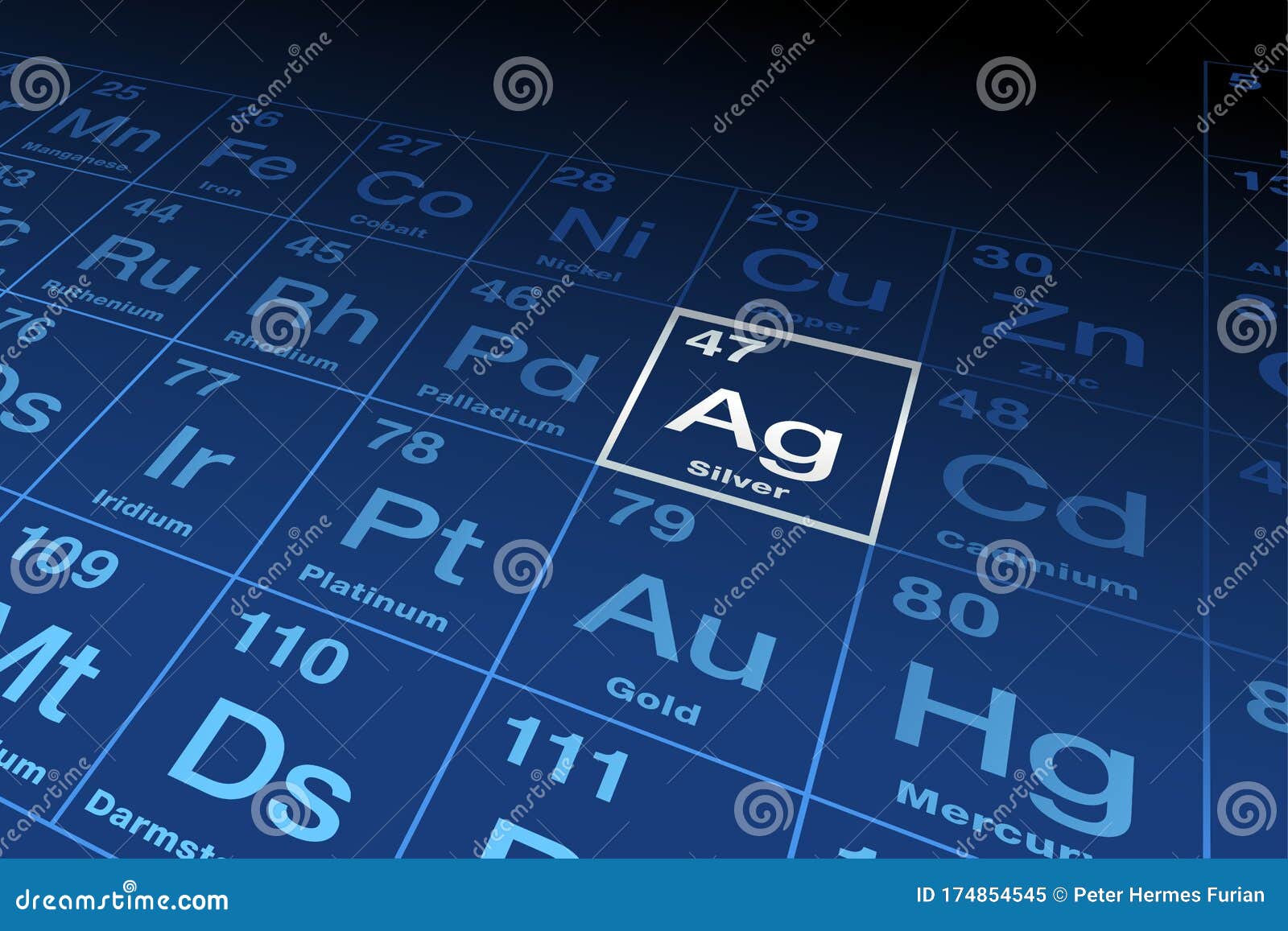
Silver and silver compounds have many uses. This is a list of the 118 chemical elements that have been identified as of 2023. Silver can be obtained from pure deposits, from silver ores such as argentite (Ag 2S) and horn silver (AgCl), and in conjunction with deposits of ores containing lead, gold or copper. Therefore, there are various non-equivalent definitions of atomic radius.Archaeological evidence suggests that people have been using silver for at least 5000 years.
#SILVER ON PERIODIC TABLE FREE#
However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. An adult human needs around 1.2 milligrams of copper a day, to help enzymes transfer energy in cells. It must be noted, atoms lack a well-defined outer boundary. The atomic radius of Silver atom is 144pm (covalent radius). Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance. The atomic mass is carried by the atomic nucleus, which occupies only about 10 -12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. Mass numbers of typical isotopes of Silver are 107, 109. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. The difference between the neutron number and the atomic number is known as the neutron excess: D = N – Z = A – 2Z.įor stable elements, there is usually a variety of stable isotopes. Neutron number plus atomic number equals atomic mass number: N+Z=A. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10 -19 coulombs. Silver is a soft, white, lustrous transition metal, it exhibits the highest electrical conductivity, thermal. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. Silver is a chemical element with atomic number 47 which means there are 47 protons in its nucleus. Atomic Number – Protons, Electrons and Neutrons in Silver


 0 kommentar(er)
0 kommentar(er)
